The correct order of the spin-only magnetic moments of the following complexes is : - Sarthaks eConnect | Largest Online Education Community
![The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube](https://i.ytimg.com/vi/KVj56QvOV1I/maxresdefault.jpg)
The spin-only magnetic moment of `[Fe(NH_(3))_(6)]^(3+) and [FeF_(6)]^(3-)` (in units of BM ) - YouTube
56.Spin only magnetic moment of Mnx+ ion is root 15B.M.Then what is tge value of X OPTIONS:A)6 B)4 C)2 D)8

Write the spin only formula and give the unit of magnetic moment - Chemistry - Structure of Atom - 15663443 | Meritnation.com
![The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are](https://d10lpgp6xz60nq.cloudfront.net/ss/web/618499.jpg)
The spin-only magnetic moments of [Mn(CN)(6)]^(4-) and [MnBr(4)]^(2-) in Bohr Magnetons, respectively, are
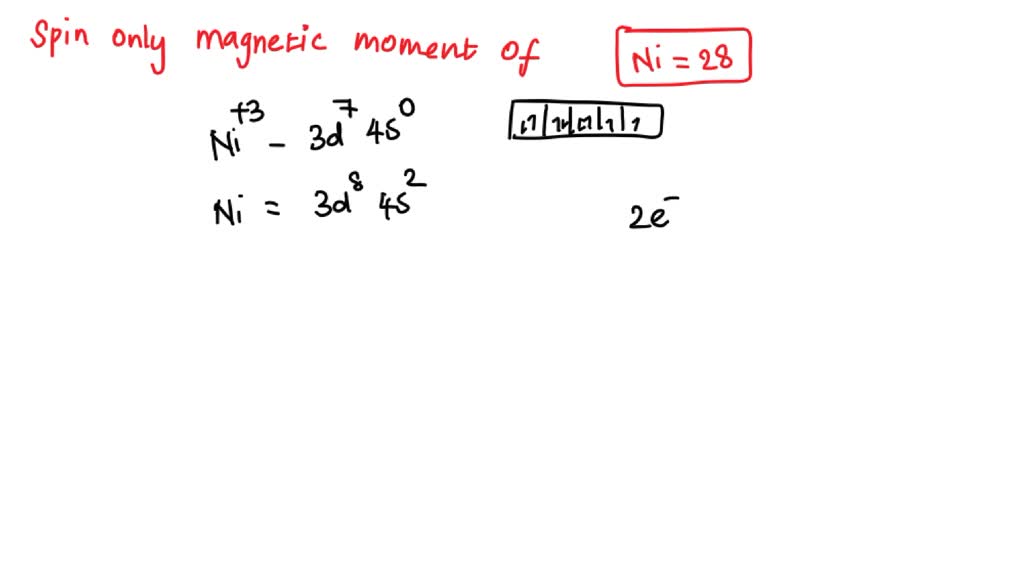
SOLVED: What is value of spin only magnetic moment of Ni (Z=28) in +3 oxidation state ? a)2.8 BM b) 3.1 BM c)0.0 BM d) 1.7 BM
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 BM. The correct one is - Sarthaks eConnect | Largest Online Education Community
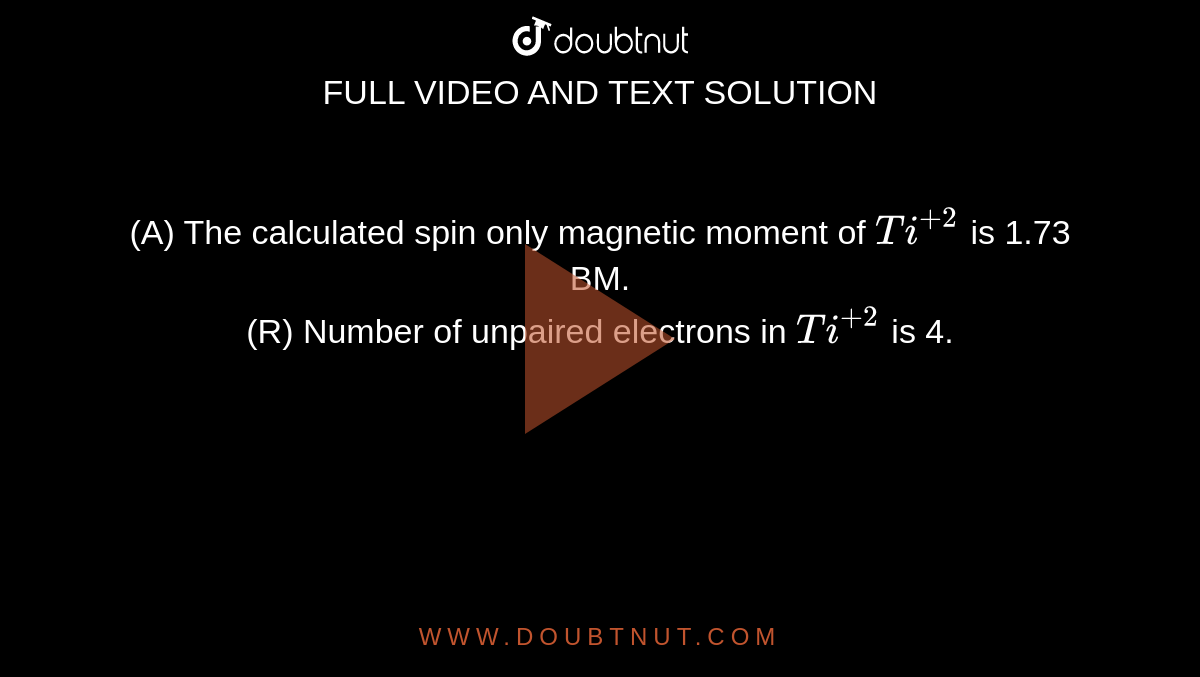


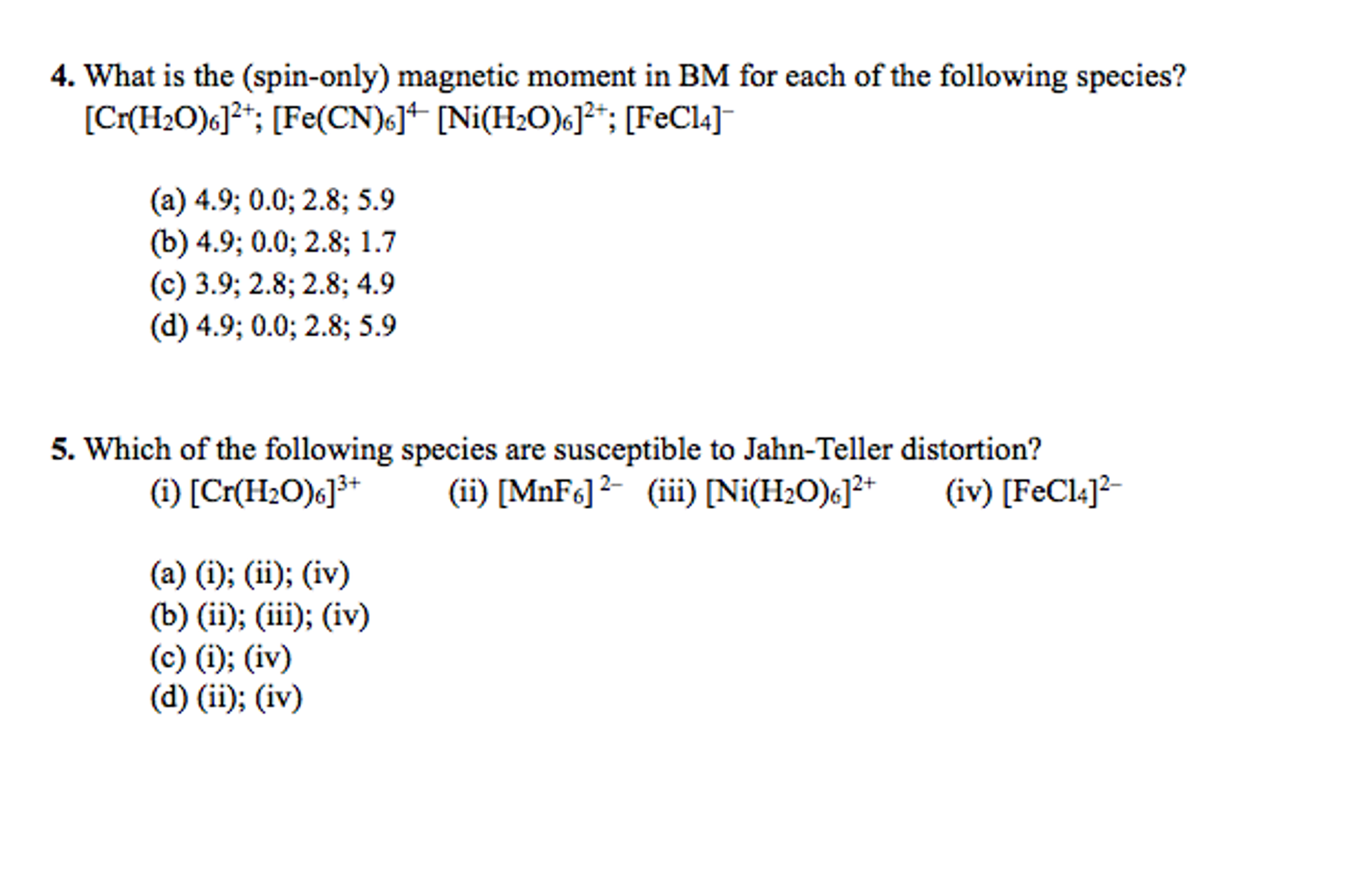
![The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab The spin only magnetic moment of [CrF₆]⁴⁻ (atomic number for Cr is 24) is - NEETLab](https://neetlab.com/wp-content/uploads/2017/11/The-spin-only-magnetic-moment-of-CrF-atomic-number-for-Chemistry-Question-.jpg)







![Spin only magnetic moment of the compound Hg[Co(SCN)4] is : Spin only magnetic moment of the compound Hg[Co(SCN)4] is :](https://d1hhj0t1vdqi7c.cloudfront.net/v1/VzQyTnk4eDZrYms=/sd/)